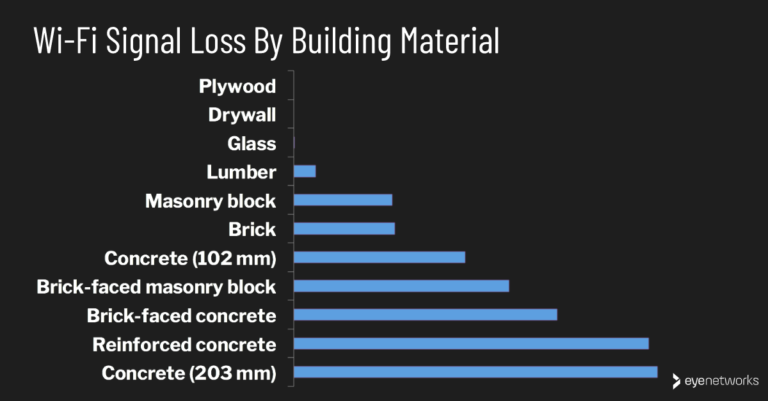
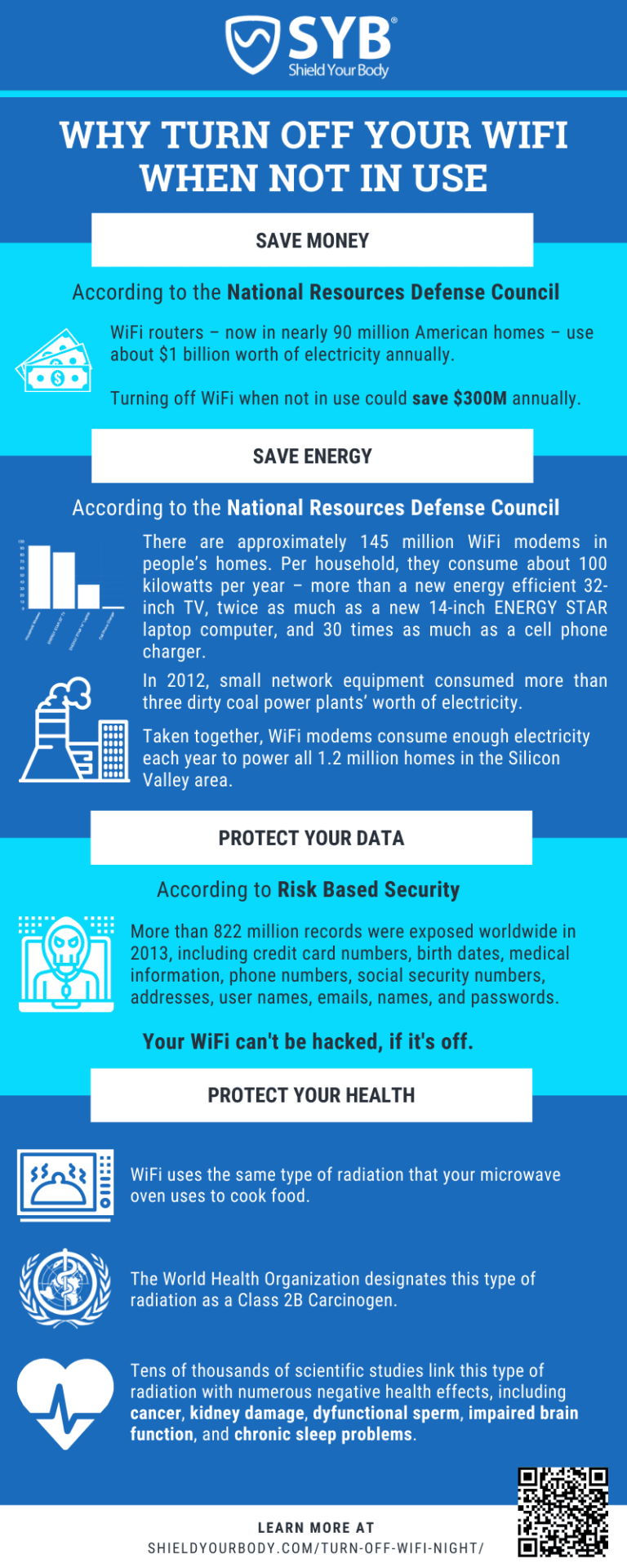
What Are The 3 Properties Of Matter?
Matter is the physical material that makes up the universe and everything in it. All matter has three basic properties: mass, volume, and shape. Mass is the amount of matter in an object, volume is the amount of space an object occupies, and shape is the form of an object. Each property plays an important role in identifying and classifying matter.
Definition of Matter
Matter is the physical substance of which the universe is composed. It is the stuff that makes up our world, from the smallest particles of atoms to the largest galaxies. Matter can be divided into three distinct properties, namely mass, volume, and density. Mass is the amount of matter contained in an object, volume is the amount of space an object takes up, and density is the mass per unit volume. These three properties can be used to determine the physical characteristics of a substance, as well as its behavior. For example, mass will determine the amount of force necessary to move an object, volume will determine how much space an object takes up, and density will influence how an object behaves when in contact with other objects. Understanding the three properties of matter is essential in understanding the behavior of matter in our universe.
Physical Properties of Matter
Matter is a fundamental concept in science that can be studied in various ways. It’s important to understand the basic properties of matter, which include mass, volume, and density. These three properties of matter are used to describe and classify different types of materials. Mass is a measure of how much matter is present in an object or sample, while volume is the amount of space an object occupies. Density is a measure of the concentration of matter in a given area.
In order to better understand the physical properties of matter, it’s important to understand the relationship between these three properties. Mass and volume are directly related, meaning that when one increases, the other increases as well. Density, however, is inversely related to both mass and volume. That is, when mass or volume increases, density decreases.
The physical properties of matter are important to understand because they are used to describe and classify different types of materials. For example, a material’s density can help determine its suitability for a particular purpose. By understanding the three properties of matter, scientists can gain a better understanding of the materials they are working with and how they interact with each other.
Chemical Properties of Matter
Matter is the physical material that makes up the universe, and its components are found in a variety of forms. Each form has its own set of unique properties, the three most common of which are chemical properties, physical properties, and structure. In this blog post, we’ll be focusing on chemical properties, which are defined as the characteristics of a substance that can be measured or observed during a chemical reaction. This includes reactivity, reactivity to heat, reactivity to light, and reactivity to pressure.
Chemical properties can vary greatly from one form of matter to another. For example, metals like iron and aluminum are highly reactive in the presence of air and water, while carbon-based compounds like oil and gas are relatively inert. The reactivity of a substance also affects its other properties, so understanding the chemical properties of a substance can help us understand its physical and structural properties as well. In addition, chemical properties can be used to identify different types of matter and to predict the outcome of chemical reactions.
In conclusion, chemical properties are one of the three main properties of matter, and they can be used to identify different types of matter, predict chemical reactions, and even understand the physical and structural properties of a substance. Understanding these properties allows us to better manipulate and understand the materials we encounter in our everyday lives.

States of Matter
, Chemical Composition, and Physical Properties.
Matter is the physical material that makes up the universe. It’s the stuff that our bodies, other living things, and all the objects around us are made of. It exists in various forms, and understanding these forms and their properties is essential to understanding the universe. All matter has three main properties: states of matter, chemical composition, and physical properties.
States of matter include solids, liquids, and gases. Solids have a fixed shape and volume, while liquids and gases take the shape of their container. Chemical composition refers to the arrangement of atoms and molecules that comprise the substance. Physical properties are characteristics that can be observed or measured without changing the substance’s chemical composition. Examples of physical properties include density, color, odor, and electrical conductivity.
By understanding the three properties of matter, we can gain a better understanding of the materials that make up our world. This knowledge can be used to develop better products and technologies, as well as to better understand the universe around us.
Examples of Physical and Chemical Properties of Matter
Matter is everything that takes up space and has mass. But matter can be broken down into three main categories – physical properties, chemical properties, and atomic properties. Physical properties involve the physical characteristics of matter, such as size, shape, color, density, and boiling point. Chemical properties refer to how matter reacts with other substances, such as its ability to react to a particular catalyst or its flammability. Finally, atomic properties involve the structure of matter at the atomic level, such as the number of protons and neutrons in an atom.
Understanding the three properties of matter is important for many fields, from chemistry to engineering. Physical properties of matter can be observed and measured without changing the identity of the substances. Examples of physical properties include melting point, density, color, and boiling point. Chemical properties involve the ability of one substance to react with another, such as its flammability, reactivity, and solubility. Atomic properties involve the structure of matter at the atomic level, such as the number of protons, electrons, and neutrons in an atom.
By understanding the three properties of matter, we can better understand how the world works and how different substances interact. The physical and chemical properties of matter allow us to study the structure and properties of the substances, while the atomic properties allow us to study the structure of the atoms that make up the matter. Understanding the three properties of matter can help us understand the world around us and make informed decisions about how to best use the resources available.
Conclusion
Matter is the physical material that makes up the universe. It displays three distinct properties – mass, volume, and density – that can be used to identify and define different types of matter. Mass is the amount of matter present, while volume is the amount of space that matter occupies. Density is the mass per unit of volume. Knowing the properties of matter is important for scientists, engineers, and researchers in many fields. It can be used to identify different types of matter, measure and compare them, and better understand the physical universe.
FAQs About the What Are The 3 Properties Of Matter?
Q: What are the three properties of matter?
A: The three properties of matter are mass, volume, and density. Mass is the amount of matter in an object, while volume is the amount of space the object occupies. Density is the mass per unit of volume.
Q: How are the three properties of matter measured?
A: Mass is typically measured in grams or kilograms. Volume is usually measured in cubic centimeters, cubic meters, or liters. Density is typically measured in grams per cubic centimeter or kilograms per cubic meter.
Q: What is the difference between mass and weight?
A: Mass is a measure of the amount of matter in an object, while weight is a measure of the force of gravity on an object. Mass is constant regardless of the environment, while weight can change depending on the environment.
Conclusion
In conclusion, the three properties of matter are mass, volume, and density. Mass measures the amount of matter in an object, volume measures the amount of space an object takes up, and density measures the amount of matter in an object per a given amount of space. These three properties are the fundamental characteristics of matter that allow us to understand the physical behavior of different substances.